
Shreemanta K Parida
Justus-Liebig University, Germany
Title: ATMP Cell Therapy: Triumphs and constraints for unmet clinical needs
Biography
Biography: Shreemanta K Parida
Abstract
Immunotherapy for Cancer, adjudged "Breakthrough of the year 2013" by journal "Science", was a paradigm shift and "game changer" in the conquest of cancer by targeting immune-system than the tumour. Cell therapy and immune therapy have opened up plethora of options to address unmet clinical needs with greater role in haemato-oncology field with promising clinical success in combating graft vs. host disease in transplant patients as well as in preventing infections in them. However, achieving the optimal numbers and the desired phenotypes of cells by conventional expansion culture methods have been major constraints. The increasing use of cell therapy towards myriads of clinical conditions has led to production processes in accordance with Good Manufacturing Practice (GMP) for Advanced Therapeutic Medicinal Product (ATMP). In cellular therapy, safety remains of paramount importance and refers to consistency, quality and potency, not only at the batch release level but also during the process development which should be adapted to closed systems that are easy to use. Implementing dynamic controls during the manufacturing of clinical-grade cells for therapy is essential to ensure microbiological safety and to avoid potential adverse effects linked to genomic instability driving transformation and senescence or decrease of cell functions (immunoregulation, differentiation potential). To meet this growing need of producing required cells of choice in bulk from bone marrow, peripheral blood or other tissues consistently in quality and numbers in a controlled, reproducible, robust, and efficient dynamic environment, tools and technologies have evolved offering in the form of GMP certified closed system at affordable cost to advance cell therapy into practice for the unmet clinical needs. We are at an interesting crossroad to push the limits making it accessible for the best outcomes for patients in precision medicine by validating tools and technologies as well as performing robustly designed clinical trials as way forward.
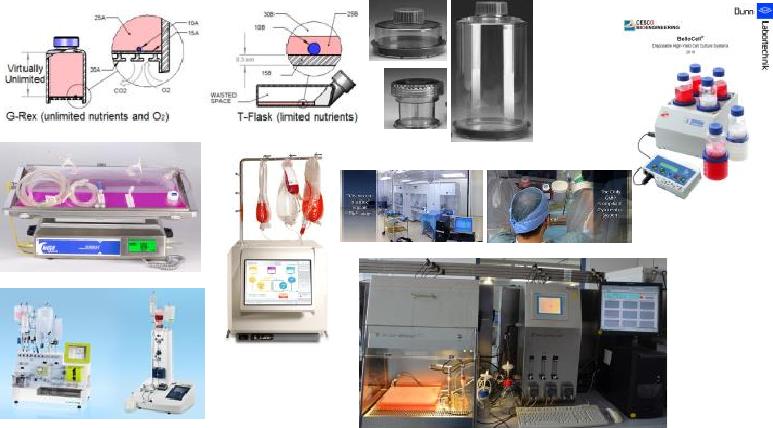
An Overview of Manufacturing possibilities for ATMP Cell Therapy




