Day :
- Vaccine Delivery Systems and Adjuvants | Vaccine Production and Delivery Technologies | Hepatitis, HIV and STD Vaccines | Vaccination for Emerging and Re-emerging Diseases | Cancer Vaccines
Location: Sylt 1-2

Chair
Giulio Tarro
Foundation de Beaumont Bonelli for Cancer Research, Italy

Co-Chair
Yusuf Omosun
Morehouse School of Medicine, USA
Session Introduction
Yusuf Omosun
Morehouse School of Medicine, USA
Title: VCG modulate innate and adaptive immunity to vaccine antigens
Time : 11:05-11:30

Biography:
Yusuf Omosun is involved in research aimed at understanding how Chlamydia infection increases the risk of developing tubal factor infertility (TFI), and finding ways to ameliorate this process through more efficient diagnosis or the discovery of viable vaccines. This would have a tremendous impact in women's reproductive health by reducing the detrimental pathology of Chlamydia.
Abstract:
Statement of the Problem: Vaccination strategies utilizing subunit antigens often rely on the incorporation of effective adjuvants to modulate immune responses. Vibrio cholerae ghosts (VCGs; genetically derived empty V. cholerae cell envelopes) constitute an effective delivery system that promotes the induction of protective immunity in the absence of external adjuvants. However, the mechanism by which VCGs enhance immunity has not been elucidated. We hypothesized that the immunostimulatory ability of VCGs involves dendritic cell (DC) activation and function.
Aim: Aim of this study is to evaluate the immunomodulatory effect of VCGs on induction of innate and adaptive immune responses.
Methodology & Theoretical Orientation: Mouse bone marrow-derived DCs (BMDCs) co-cultured with VCG or UV-irradiated Chlamydia elementary bodies (UV-EBs) were stained with monoclonal antibodies against co-stimulatory molecules and surface expression was analyzed by flow cytometry. The magnitude of cytokines secreted by culture supernatants or splenocyte-stimulated cultures was analyzed by cytokine ELISA. Furthermore, the ability of VCG-pulsed DCs to present chlamydial antigen to infection-sensitized CD4+ T cells and enhance the protective immunity of chlamydial antigens was also evaluated.
Results: VCG-pulsed DCs showed increased secretion of proinflammatory cytokines and expression of co-stimulatory molecules associated with DC maturation in response to stimulation with UV-EB. Also, co-culture of VCG with DCs resulted in effective chlamydial antigen presentation and enhancement of protective immunity.
Conclusion & Significance: These results demonstrate that VCGs activate the maturation of DCs leading to enhancement of innate and adaptive immunity to a co-delivered antigen. The results highlight the potential of the VCG as immunomodulators for enhancement of protective immunity against microbial infections.
Qing He
Morehouse School of Medicine, USA
Title: The emerging role of ASC in dendritic cell metabolism and function during chlamydia infection
Time : 11:30-11:55

Biography:
Qing He is an Associate Professor of Microbiology, Biochemistry & Immunology in the Morehouse School of Medicine, USA. She has a broad background in “Infectious disease and host innate and acquired immune responses” with specific training and expertise in key research areas. Her work focuses primarily on “Antigen processing, presentation and immunomodulation”. She focuses on “The role of apoptosis-associated speck-like protein containing a CARD (ASC) in GT dendritic cell (DC), macrophage and T & B cell functions during genital chlamydia infection and to investigate the metabolic and immunologic plasticity of mucosal dendritic cells and T cells in the absence of the inflammasome. She has published several manuscripts that are related to this project.
Abstract:
Chlamydia trachomatis is a bacterial agent that causes sexually transmitted infections worldwide. The regulatory functions of dendritic cells (DCs) play a major role in protective immunity against chlamydia infections. The mechanisms underlying this immunomodulation are not fully understood. The inflammasome adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC) regulates the direction of immunity against such bacterial infections. Here, we investigate whether ASC, the critical components of inflammasome activation, participate in regulation of DC activation and function and the possible mechanisms during chlamydia infection. We observed that following chlamydia stimulation, the maturation and antigen presenting task of ASC-/- DCs were impaired compare to wild type DC (WT DC). Also, ASC deficiency induces a tolerogenic phenotype in chlamydia stimulated DCs, which may induce immune pathological response in infected host. We observed that following chlamydia stimulation of ASC-/- DCs prevented the chlamydia-induced increase in aerobic glycolysis, measured as extracellular acidification rates (ECARs) and had significantly reduced pyruvate production from the metabolism of glucose during glycolysis. To determine the effect of this reduction in pyruvate production in cellular respiration, we determined the morphology of the mitochondria. The results revealed that the mitochondria of infected ASC-/-DCs had their cristae disrupted compared to the normal narrow pleomorphic cristae found in un-stimulated WT, ASC-/- and stimulated WT DCs. In conclusion, the results suggest that ASC deficiency interrupts DC function through reprogramming of DC metabolism starting from glycolysis to the electron transport chain which occurs within the mitochondria, which controls the actions and functions of DCs during chlamydia infection. The interface between ACS and metabolism in innate immunity is of great interest. It may be possible for small molecules to reprogram the metabolism of immune cells to enhance vaccine efficacy against infectious diseases and tumors.
Gustavo Cabral de Miranda
University of Oxford, UK
Title: The use of new adjuvants and VLPs for the development of vaccines for emerging and infectious diseases
Time : 11:55-12:20

Biography:
Gustavo Cabral de Miranda is a Post-doctoral Research Scientist in Vaccinology at Jenner Institute, University of Oxford, UK. He has a Degree in Biological Science at Bahia State University; MSc in Immunology at Federal University of Bahia and; PhD in Immunology at University of São Paulo, Brazil. He is currently pursuing his research which focuses on The use of new adjuvants, especially microcrystalline tyrosine (MCT) and phosphatidylserine (PS) derivatives, and virus like particles (VLPs) for the development of vaccines against malaria and Flavivirus, especially Zika virus (ZIKV) and Dengue virus.
Abstract:
The increased burden of neglected and emerging infectious disease (EID) has caused a serious global public health threat. According to World Health Organization (WHO), infections such as dengue fever, Zika virus (ZIKV) and malaria are associated with billions of deaths worldwide. For disease prevention and control, vaccination remains the most effective tool. The use of adjuvants in vaccine development is a well-established concept and practice. Adjuvants improve and modulate immune responses to the desired antigen. They increase the half-life of vaccine antigens and improve antigen uptake, processing and presentation by APCs (antigen-presenting cells). Micro-crystalline tyrosine (MCT) is a depot adjuvant formulated in licensed vaccines for use in humans with an excellent safety profile. Virus-like particles (VLPs) have a high capacity to induce strong humoral and cellular immune responses and may have the potential to increase vaccine efficacy against malaria and ZIKV, in particular if combined with MCT. We investigated the impact of MCT and/or VLP presented vaccine antigens and compared to protein antigens formulated with alum. We have demonstrated that MCT is able to produce high and sustained IgG responses that are specific and protective against the sporozoite of P. vivax. Our results showed that malaria antigens conjugated to VLPs and formulated in MCT induced higher antibody and T-cell responses and protected against Plasmodium bergei/vivax. Hence, combining MCT with VLP-conjugate vaccines defines a promising strategy for the development of protective malaria vaccines. We are also investigating the use of VLPs for ZIKV vaccine development. We have induced high antibody titres using domain III (DIII) of the envelope (E) protein of ZIKV. This protein is strictly associated with specific virus neutralization, therefore this vaccination strategy should avoid eliciting cross-reactive antibodies against other Flavivirus, which have been shown previously to have a detrimental effect upon infection with other Flavivirus e.g. Dengue.
Imran Saleem
Liverpool John Moores University, UK
Title: Pulmonary delivery of a mucosal nanocarrier vaccine for Pneumonia
Time : 12:20-12:45

Biography:
Imran Saleem is a Reader in Nanomedicine at Liverpool John Moores University, UK. His research interest includes “Developing novel delivery systems for targeting therapeutic agents to their site of action, with particular emphasis on lung diseases via dry powder pulmonary delivery”. He has over 10 years of experience in the area of Micro/Nanoparticle Formulation and Drug Delivery Systems, and has published extensively in peer-reviewed journals, conference abstracts and book chapters. His research group is focused on “The design and development of carriers for delivery of bio-macromolecules including vaccines and drugs”. Currently, his research group is working on the design and development of nanocarriers for pulmonary delivery of pneumococcal vaccine, gene delivery for treatment of COPD and lung cancer. He is currently investigating nanoparticle (NPs) delivery systems and manufacturing nanocomposite micro-particle carriers (NCMPs) for pulmonary delivery.
Abstract:
Statement of the Problem: There is a huge drive in the vaccine research field, pharmaceutical industry and Bill Gates Foundation for effective targeting of dendritic cells (DCs) to enhance the immune response and for needle-free vaccination. The pulmonary route has an abundance of antigen presenting cells (such as macrophages, DCs) for targeting vaccines. Furthermore, nanoparticles (NPs) due to their size can target DCs enhancing the immune response. However, NPs have poor aerosolization performance as dry powders.
Aim: The aim of this study was to compare encapsulated and adsorbed pneumococcal protein (PspA), onto poly(glycerol adipate-co-ω-pentadecalactone), PGA-co-PDL, NPs to target lung DCs. Further to formulate these NPs into dry powder, nanocomposite microparticles (NCMPs) were suitable for pulmonary vaccine delivery.
Methodology & Theoretical Orientation: NPs were prepared using an emulsion solvent evaporation method and PspA was adsorbed (F1) onto the surface of NPs or encapsulated (F2) (100: 20 [NP: PspA]). F1 and F2 were spray-dried in an aqueous suspension of leucine (1:1.5) to produce NCMPs and characterized in terms of particle size, loading, cell viability, protein stability (SDS-PAGE), integrity (circular dichroism, CD), antigenicity (ELISA), aerosolization studies and lung immunization in mice.
Conclusion & Significance: F1 and F2 produced similar size NPs but the PspA loading was significantly greater in F2 (310.4±25.3 nm, 65.73±5.6 µg/mg) compared to F1 (322.83±4.25 nm, 19.68±2.74 µg/mg). F1 had FPF% >75%. The NPs appear to be well tolerated by DCs cell lines (F1 and F2 NPs ≥90% cell viability) at 19.5 µg/mL after 4 h exposure. The antigenicity (>95%) confirmed that PspA was stable in both formulations after spray-drying. F1 induced an earlier control of the infection with lower bacterial load in the lungs after challenge. The results provide an indication that it may be feasible to use these NPs/NCMPs carriers containing protein antigens for pulmonary vaccine delivery against lung infection with pneumococci.

Nadia Tagnaouti
Precision NanoSystems Inc., Canada
Title: Next-generation millisecond manufacture of genetic vaccines and lipid/polymer-based vaccines
Time : 13:45-14:15
Biography:
Nadia Tagnaouti, Regional Manager Central Europe, Eastern Europe and Middle East, Precision NanoSystems Inc., Canada.
Abstract:
Lipid nanoparticles, liposomes and polymer-based nanoparticles are widely used to deliver vaccines. Here we present a platform for the millisecond fast manufacturing of genetic vaccines and lipid/polymer-based vaccines. We will also describe how, by fine-tuning the size, composition and surface moieties of vaccine particles, you can optimize efficacy and immune response. Examples of lipid- and polymer-based nanoparticle systems manufactured with the NanoAssemblr platform will be described.
How this robust platform allows you to manufacture large batches of vaccines in GMP environment to meet both the needs of large-scale production and small-scale personalized cancer vaccines.
Erwann Loret
Aix-Marseille University, France
Title: A reasonable hope to cure HIV with the Tat Oyi vaccine: Results and follow up of a double blinded randomized phase I/IIa clinical trial in France
Time : 14:15-14:40

Biography:
Erwann Loret obtained is Ph. D. in France in February 1990, then he went to USA for a first post-doc in the department of biophysics and biochemistry at Oregon State University, followed by a second post-doc in the department of chemistry at the University of California, Davis. He was hired in 1992 in the Centre National de Recherche Scientifique(CNRS), which is the main French national agency for scientific research. Erwann Loret obtained the GlaxoSmith Kline Drug Discovery and Development Award from an American scientific committee for his contribution to HIV research, the Medail of honor from the Aix Marseille University (AMU) and the French National Institute for Intellectual Properties Award. He is heading the ETRAV lab that is the only lab working on HIV at Marseille. He is member of the Editorial Board of scientific journals and author and co author of 60 scientific publications with a high impact factor and five patents with PCT. His main field of interest is related to the HIV-1 Tat proteins. He is the inventor of the Tat Oyi vaccine and coordinated the EVATAT clinical trial.
Abstract:
We know that it was possible to cure from HIV for at least one patient called the “Berlin patient”. Although the hematopoietic stem cell transplantation that saves him was not reproducible, his cure from HIV was well documented and shows that two steps are required. The first step is that the HIV DNA in peripheral blood becomes and stays undetectable. The second step is a significant decrease of antibodies against HIV-1 (never observed in HIV infected patients under successful cART) called retroseroconversion. BIOSANTECH was the sponsor of a phase I/II clinical trial carried out at Marseille on 48 HIV infected volunteers under cART with the Tat Oyi vaccine (Loret et al., Retrovirology 2016). These volunteers were randomized in double-blind method into four groups (n=12) and three intradermal injections were made with respectively for each group 0, 11, 33, or 99ug of a synthetic Tat Oyi protein in buffer without adjuvant at times designated by month 0 (M0), M1 and M2. The volunteers then underwent a one month cART interruption at M5. The vaccine reduces of 1.5 log copies/ml the HIV rebound median compare to the placebo group. Furthermore no increase of HIV infected cells was observed following cART interruption (p=0.001). A follow up of volunteers three years after vaccination shows that vaccinated volunteers who were HIV DNA undetectable at the end of the trial are still undetectable and the number of HIV DNA undetectable increased in all groups excepted in the placebo group (Fig 1). Preliminary results show that retroseroconversion begins in two volunteers.
HIV DNA medians in peripheral blood for each group. Volunteers with undetectable HIV DNA (< 20 copies/ 106 PBMC) were counted as having 19 copies, which is 1.3 log copies/ml (dashed line). The placebo group is depicted as a black square, the 11 μg group as a black circle, the 33 μg group as a white square and the 99 μg group as a white circle.
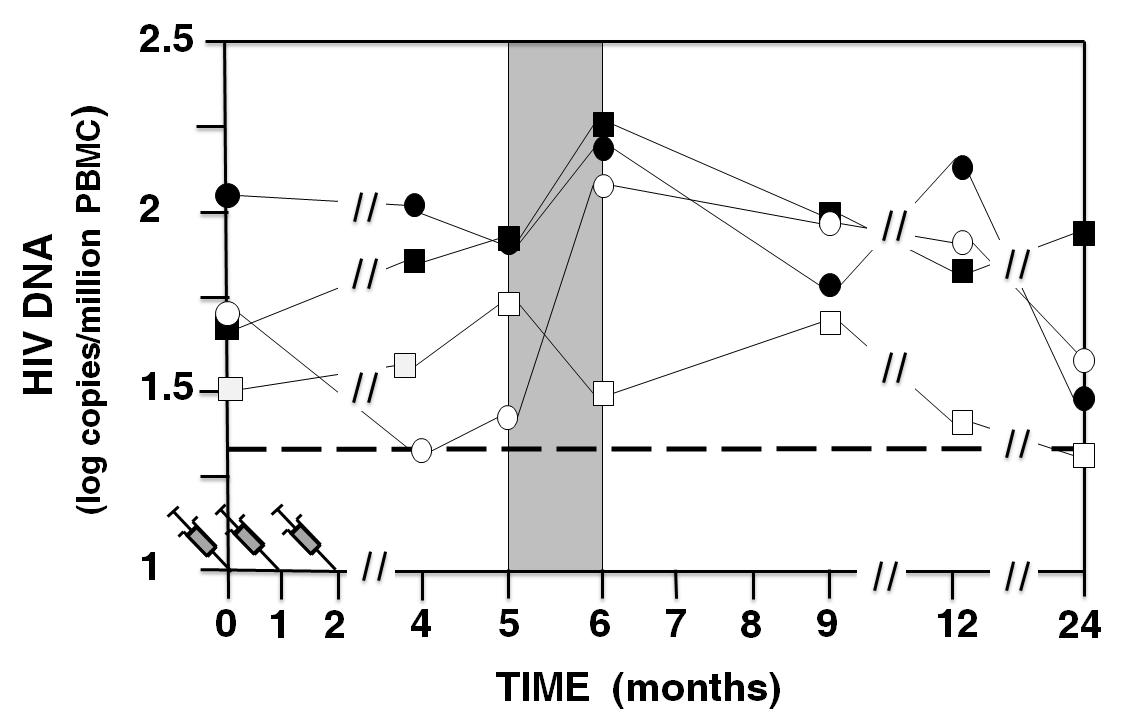
Figure 1: HIV DNA medians in peripheral blood for each group.
Cheng He
China Agricultural University, China
Title: Evaluation of nanoparticle-delivered inactivated whole antigen against Chlamydia psittaci infection in SPF chickens
Time : 14:40-15:05

Biography:
Cheng He completed his Bachelor of Veterinary Medicine at Beijing Agricultural University in 1990. As a Visiting Scholar, he studied at Manchester University, Moredun Research Institute in 2005 and Morehouse School of Medicine, USA in 2013. He focuses on “Epidemiology, infection and immunity of chlamydiosis”. In addition to more than 40 peer-reviewed international publications, he was funded four projects by NSFC, five projects by Ministry of Science and Technology (MoST), China, two projects by Beijing National Natural Science Foundation, two PhD dissertations by Ministry of Education, China and three projects by National Bureau. Finally, he got one vaccine certificate against C. psittaci using recombinant MOMP as vaccine candidate and registered Salmovir against pigeon NDV and salmonellosis for Poland. With respect to his innovations, he received 16 Chinese patents.
Abstract:
Avian Chlamydia psittaci (C. psittaci) is threating to poultry industry as well as the closely contacted humans due to highly prevalence and lack of the commercial vaccine. A novel nanoparticle vaccine with the cellular-elicited adjuvant was prepared to achieve a better immune response against C. psittaci infection and it included a gel-formed or a microsphere-formed chitosan as a deliver particle, standard CPG or VCG as the adjuvant and the inactivated elemental bodies of C. psittaci. Total of 105 SPF chickens aged 7-day old was divided into seven groups, 15 birds per group. Chickens received the nanovaccine with CpG or VCG adjuvant by intranasal administration or by intramuscular route. Meanwhile, birds were inoculated with r-MOMP, or inactivated whole EB or gel chitosan or microsphere chitosan as the control group. All above groups were immunized and boosted with 14 day interval. Post immunization, C. psittaci specific antibodies were detected weekly. After boosting, lymphocyte proliferation, T cell subsets, cytokines were monitored using commercial kits. Finally, birds were challenged with 1×108 IFU/ml live EBs of virulent C. psittaci via larynx inoculation and chlamydial shedding were determined using cell culture. Post prime-boost strategy, birds with VCG-adjuvant nanoparticle induced an increasing-trend antibody and no statistical difference was found as compared to rMOMP vaccine. However, VCG-adjuvant nanoparticle yielded a highly splenic lymphocyte proliferation, CD4+/CD8+ ratio, as well as significant elevation of IFN-γ, IL-2 and IL12p40 in lung lavage fluids. Moreover, a reducing trend of chlamydial shedding was found in the birds with VCG-adjuvant nanoparticle. Lesion index in lungs and air sacs were also reduced in the birds with the VCG-adjuvant nanoparticle vaccine. Taken together, VCG-adjuvant nanoparticle vaccine via mucosal immunization is a promising approach against C. psittaci infection.
Talin Barissani-Asenbauer
Medical University of Vienna, Austria
Title: Mucosal Immunization via conjunctiva: Where do we stand?
Time : 15:05-15:30

Biography:
Talin Barisani Asenbauer is an Associate Professor of Ophthalmology at Medical University of Vienna. She succeeded to obtain one of eight highly competitive peer-reviewed Laura Bassi Centre of Expertise grants from the Austrian Ministry of Economics allowing her to establish in 2010 with her industrial partners the Laura Bassi Centre of Expertise OCUVAC at the Center of Pathophysiology, Immunology and Infectiology, Medical University of Vienna. OCUVAC aims at achieving a multidisciplinary understanding of trachoma and ocular immunity that underpins the more translational research in the centre, while having the potential for the discovery of innovative drug-delivery systems and ocular vaccines. Her research interests include “Rare and neglected ocular diseases, uveitis, ocular immunology, inflammation & infection, drug delivery to ocular tissues and ocular vaccine development.
Abstract:
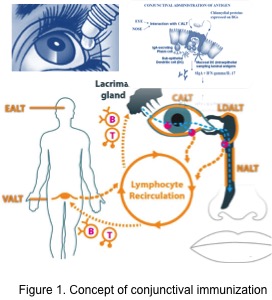
The ocular surface is recognized as part of the mucosal immune system as conjunctiva-associated lymphoid tissue (CALT). This lymphoid tissue consists of intraepithelial lymphocytes, sub-epithelial lymphoid follicles (conjunctival follicles) and adjacent lymphatic and blood vessels. Furthermore, together with lacrimal drainage-associated lymphoid tissue (LDALT), CALT forms the eye-associated lymphoid tissue (EALT). All these components have a key role in the protection of the ocular surface by initiating and regulating immune responses. In the context of needle-free delivery approach, the conjunctiva and its underlying CALT, with its possibility to detect antigens, taken up at the ocular surface, present them, and generate specific and nonspecific effector cells, would be an attractive choice for mucosal immunization, particularly against ocular infections. As the conjunctiva and CALT are interconnected with the nasal mucosa via the draining tear duct, antigens would additionally drain to nasal-associated lymphoid tissue (NALT). Actual developments and outlooks for conjunctival immunization will be presented.
Aleksandra Inic-Kanada
Center of Ocular Inflammation and Infection, Austria
Title: Use of corpuscular adjuvants for ocular mucosal immunization as a strategy for vaccine against trachoma
Time : 15:30-15:55

Biography:
Aleksandra Inic-Kanada has her expertise in “Innate and acquired resistance to infection, mucosal immunity, pathogen immune modulation, autoimmunity, animal models and vaccine development”. In 2011, she joined the group of Talin Barisani-Asenbauer in Laura Bassi Center of Excellence-OCUVAC at Medical University Vienna, which aims at achieving a multidisciplinary understanding of ocular immunity and the development of a vaccine against Chlamydia trachomatis.
Abstract:
Statement of the Problem: Trachoma is the world's most common cause of preventable blindness from infectious origin and is prevalent in most rural areas throughout the developing world. It has been classified as one of the five most neglected tropical diseases.
Aim: Our overall goal is to develop an innovative, prophylactic, needle-free, safe and effective mucosal vaccine against Chlamydia trachomatis (Ct) that will prevent or reduce clinical trachoma thereby reducing chlamydia induced morbidity. The strategy employed is to reproduce and improve naturally acquired protective immunity by using a mucosal immunization strategy: eye drops mimicking the natural infection route.
Methodology & Theoretical Orientation: Immuno-proteomic profiling of sera of subjects from trachoma endemic regions enabled us to confirm the chlamydial adhesins (major outer membrane protein and polymorphic membrane proteins) as vaccine candidates. In combination with these antigens, different corpuscular adjuvants were used and tested in animal models.
Results: We have proved that eye drop vaccination irrespective of the adjuvant applied elicits higher immune response at the site of infection by blocking Ct-host cell entry with enhanced immune responses against key adhesins involved in infection.
Conclusion & Significance: The combination of relevant chlamydial adhesins and an effective adjuvant could represent a vaccine approach to achieve a protective immunity. Ocular mucosal immunization strategy might lead to a needle-free ocular mucosal vaccine suitable to overcome the current hurdles in managing trachoma and will thus have a major global impact.
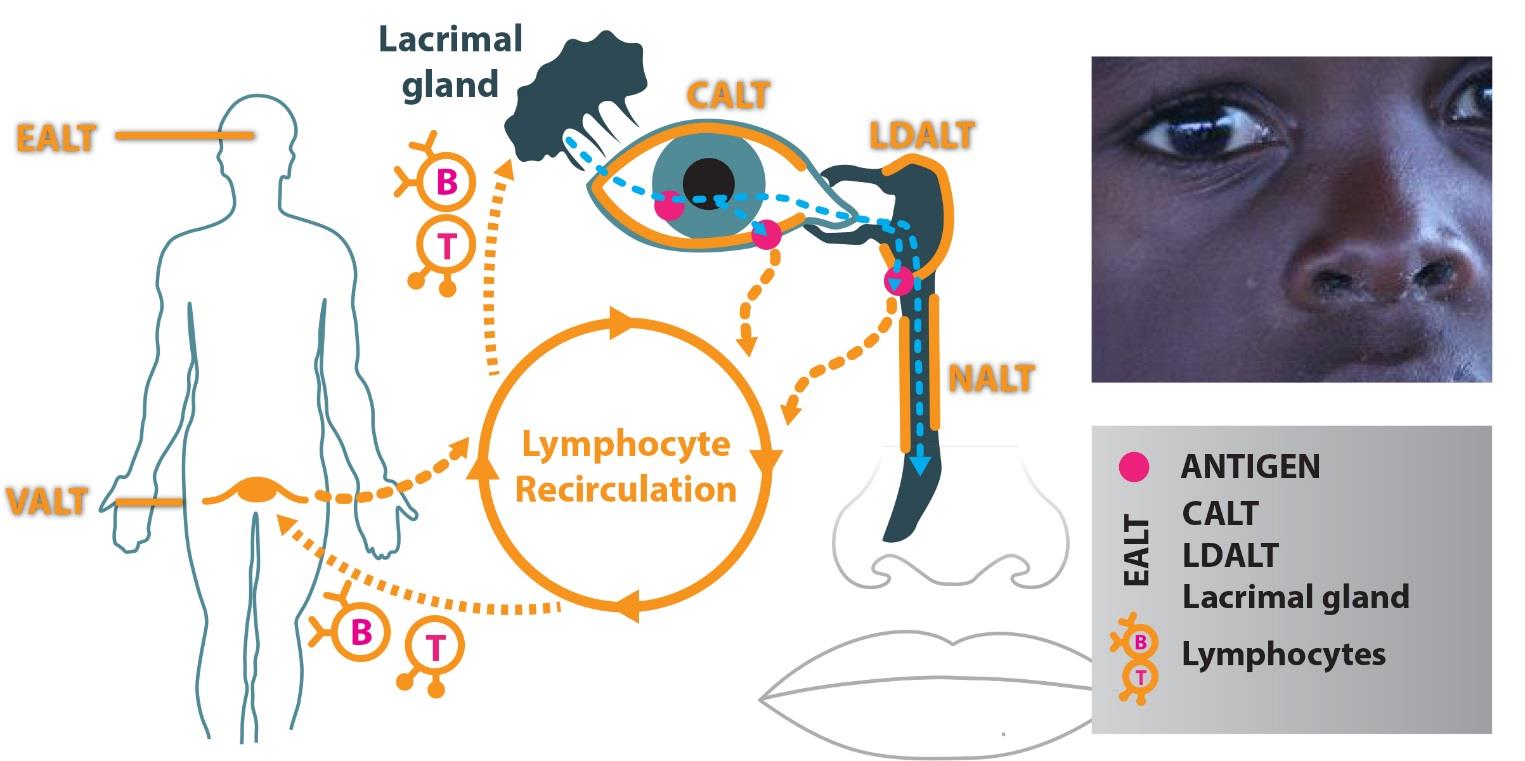
Figure 1: The eye-associated lymphoid tissue (EALT) includes the lacrimal gland, conjunctival-ALT (CALT) and lacrimal drainage-ALT (LDALT). It is a part of the immune system, which is essential to protect the organism from ocular infection with a specific IgA based immune response.
Rainer Henning
Viravaxx AG, Austria
Title: VVX001- a promising novel hepatitis B vaccine candidate
Time : 16:10-16:35

Biography:
Rainer Henning holds a PhD Degree in Organic Chemistry at Justus-Liebig-University in Giessen, Germany. He had Post-doctoral training at Colorado State University, Ft. Collins, CO, USA. He combines extensive experience in medicinal chemistry in academic and pharmaceutical industry settings with managerial achievements in the biopharmaceutical industry. His field of interest includes Medicinal Chemistry, Molecular Biotechnology with therapeutic applications in cardiology, emergency medicine, allergy and immunology and viral diseases. He is an author of 39 publications in peer reviewed journals and has given numerous presentations at chemistry and medical conferences. At Viravaxx, he serves as a CEO and directs all research activities by the company.
Abstract:
Background: HBV infection remains a serious global health challenge. 5-10% of vaccines with available vaccines do not achieve seroconversion. 350 million patients worldwide live with chronic HBV infections. New vaccine designs are required to tackle these important challenges.
Aim: Characterization of the protective effect of HBV PreS fusion proteins contained in the clinical stage allergy vaccine BM32 with respect to protection against infection with HBV.
Methods: Epitope mapping of the antibody response elicited by BM32 in rabbits and patients was performed using a collection of overlapping 30-mer peptides derived from PreS1. The sera from these animals and patients were also tested for their ability to protect HepG2-NTCP cells against infection in cell culture. Outcome measures were the secretion of HBsAg and expression of HBeAg.
Results: Sera from patients and animals immunized with BM32 demonstrated selectivity towards the NTCP binding site of the large HBV surface antigen. The selectivity was stronger in humans than in rabbits. These sera protect HepG2-NTCP cells from HBV infection to a similar extent as sera from subjects vaccinated with Engerix- B. The anti HBV activity in BM32 resides predominantly in one fusion protein component, which will be developed as VVX001.
Conclusion: BM32, containing fusion proteins with PreS from HBV capped with peptides at both termini elicits a neutralizing IgG antibody response in immunized individuals. This immune response is focused on the virus attachment site and prevents virus entry into target cells. One of the components of BM32 is responsible for this effect and may be a promising HBV vaccine candidate.
- Biodefense Vaccine against Bioterrorism | Vaccines for Immune Mediated Diseases | Vaccines for Pregnant Women and Neonates | Antibodies : Engineering and Therapeutics | Animal Models and Clinical Trials
Location: Sylt 1-2

Chair
Leondios G Kostrikis
University of Cyprus, Cyprus

Co-Chair
Lallan Giri
Biologic Resources LLC, USA
Session Introduction
Lallan Giri
Biologics Resources LLC, USA
Title: Protection against bioterrorism
Time : 11:35-12:00

Biography:
Lallan Giri is currently CEO of Biologics Resources LLC (BRLLC) which is a Vaccine and Biopharma Company focused on the development of biodefense vaccines for adults and children. Professionally, he is a Vaccinologist and has made contributions to the development of several pediatric vaccines during his employment as Director of Glaxo Wellcome, as Director at Sanofi Pateur, and as Vice President at Emergent Biosolutions, Inc, and as CEO at BRLLC.
Abstract:
Bioterrorism involves the intentional release of dissemination of deadly biological agents in the cities and communities. Terrorism has become a common and easier way of causing harms to innocent public. At present time, it has caused serious concerns and insecurity in the public domain. One of the sources or methods used by terrorists is through dissemination of deadly bacteria, viruses, and chemicals. Bioterrorism agents are found typically in nature and can be grown easily. It is also possible that they can be mutated or altered to increase their ability to cause deadly diseases causing mass casualty of human lives. Anthrax letter attacks after September 11, 2001 demonstrated the ease of using the anthrax spores as a weapon of mass destruction in a bioterrorist attack. Anthrax is lethal infectious disease caused by the spore forming Bacillus anthracis. The two major virulence factors of B. anthracis are the poly-y-glutamic acid (Y-D-PGA) capsule and exotoxin. The three components of the exotoxin, Protective Antigen (PA), Lethal Factor (LF) and Edema Factor (EF) are produced and secreted separately and form two bipartite toxins that cause massive edema and organ failure. The anti-phagocytic y-D-PGA capsule disguises the bacilli from immune surveillance by macrophages and allows unimpeded growth of the bacilli leading to anthrax disease caused by both, the capsule and toxins. However, the currently licensed Anthrax Vaccine Adsorbed (AVA) or rPA based anthrax vaccines only address the toxin- induced disease, and not the capsule mediated virulence factors related disorders which contribute to the severity of the anthrax infection leading to death. The newly proposed anthrax vaccine, an antibody response triggered specially by y-D-PGA can fully neutralize the disguising capsule. However, the capsule is not immunogenic by itself and must be conjugated to a carrier protein such as PA. The conjugate vaccine concept embodies the paradigm of combining both, antibacterial (prophylactic) and antitoxin (therapeutic) components into a single vaccine. This has contributed much success in inducing protective levels of antibodies in infants and children against systemic infection with encapsulated pathogens. The development of this conjugated anthrax vaccine will fill an urgent void, delivering a well-defined and characterized biodefense countermeasure potentially suitable for infants and children. The proposed work will lead to the characterization, testing, and manufacturing of a promising conjugated anthrax vaccine candidate. This will potentially lead to the development of a conjugated anthrax vaccine with dual antigens, namely PA and PGA (polyglutamic acid).
Mark Lawrence Johnson
MJ Lawrence Consulting, Germany
Title: When funding for medical countermeasures against rare, but detrimental agents is cost-effective
Time : 12:00-12:25

Biography:
Mark Lawrence Johnson is one of the early pioneers to develop policy and procurement in the European biodefense market for medical countermeasures (MedCM). He has accrued a mature and extensive network. His professional credibility was born over 20 years ago when he began to represent leading global pharmaceutical companies such as Merck & Co., Eli Lilly, and Astellas. Following response to the European Commission’s (EC) Green Paper on CBRN preparedness in 2007, his participation at EC CBRN taskforce meetings was instrumental in getting fundamental economic elements adopted in its corresponding recommendations and action plan. He holds an MBA from Wake Forest University and a BA in Business Management from the University of Maryland. He is currently a Doctoral candidate at the Paris 2 University, Panthéon-Assas (LEMMA) working to determine economic mechanisms for achieving international availability of CBRN MedCM.
Abstract:
Some chemical, biological, radiological, and nuclear (CBRN) agents can be very damaging and pose high risk to national security because they bear potential to induce economic and social disruption. Assuming efficacious medical research and development (R&D) is executed; populations could be protected against such CBRN agents via new prophylactic drugs and vaccines or post-exposure treatment with antidotes and antimicrobials. However, the development of medical countermeasures (MedCM) against these agents is very limited. This presentation depicts specific features of the MedCM market and R&D against CBRN agents and explores the latest Ebola outbreak as an illustration of these concerns. While their R&D process and related costs are quite similar to those of commercially viable conventional diseases, it is shown that free market rewards and incentives are more uncertain and most often not sufficient for developers of MedCM to independently invest on its own since widespread dissemination of CBRN agents remains a rare and unpredictable event (e.g. few customers, low and volatile market sales potential). This results in market failures for appropriate medicines or vaccines except in the case of effective public intervention. However, priorities should be identified given the extent of such risks and the amounts of investment involved. The Ebola example is used as a case study of causal factors which led to a lack of a vaccine prior to the 2014 outbreak. In addition, possible reasons which may have triggered a re-evaluation of its prioritization as a threat worthy of high actionable concern are probed.

Susanne Rauch
CureVac, Germany
Title: An mRNA-based vaccine technology for next generation prophylactic vaccines
Time : 12:25-12:50

Biography:
Susanne Rauch works as a Scientist at CureVac where she is involved in the development of mRNA-based prophylactic vaccines. She has been trained as a Postdoc at King’s College London and as a PhD student at University Hospital Heidelberg where her work was focused on “The biology of different viruses such as HIV-1, MLV and herpesviruses.
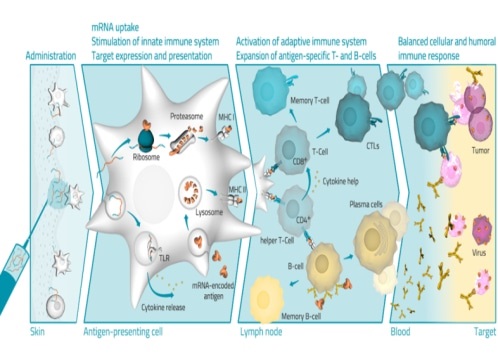
Abstract:
In recent years, messenger RNA (mRNA) based technologies have increasingly been applied in vaccine development. Such approaches have utilized mRNA for both therapeutic cancer vaccinations, and for prophylactic vaccines, drawing much attention from industrial and academic fields. RNActive®, an mRNA based vaccination technology, has yielded promising results in the development of vaccines against a variety of viral pathogens such as RSV (respiratory syncytial virus), influenza, rabies, and Ebola virus in several animal models. We have previously shown that intradermal (i.d.) application of RNActive® vaccines is able to confer protection against lethal influenza and rabies virus challenge infection in mice and induces protective levels of functional antibody responses against both viruses in domestic pigs. However, having been optimized for i.d. application, immunological responses upon intramuscular (i.m.) injection of these mRNA vaccine formulations remained less efficient as yet. Here, we describe an alternative formulation of RNActive® vaccines that is able to induce potent immune responses when applied intramuscularly using low doses (µg) of mRNA. Vaccination of mice with this RNActive® formulation encoding for influenza HA (hemagglutinin) or rabies G (glycoprotein) revealed an increase of both humoral and cellular immune responses, analyzed via functional antibody levels and ICS (intracellular cytokine staining) of T cells, respectively, compared to previous RNActive® formulations. Further experiments showed that this new vaccine formulation was able to induce potent and long lasting immune responses against influenza HA as well as high titers of rabies virus neutralizing antibodies in NHPs (non-human primates).
Shreemanta K Parida
Justus-Liebig University, Germany
Title: ATMP Cell Therapy: Triumphs and constraints for unmet clinical needs
Time : 12:50-13:15

Biography:
Shreemanta Parida is a Clinician Scientist with his expertise and passion in making a difference to patients by applying cross cutting scientific advances in patient care. He has been engaged during last 5 years in bringing ATMP cell therapy to translation in precision medicine for many unmet clinical needs. He has contributed immensely to the field of Global Health, Vaccines and Immunology over last three decades with focus on resource poor settings embracing stakeholders across disciplines. He has held many leadership positions in many top institutions in Europe, Africa and India.
Abstract:
Immunotherapy for Cancer, adjudged "Breakthrough of the year 2013" by journal "Science", was a paradigm shift and "game changer" in the conquest of cancer by targeting immune-system than the tumour. Cell therapy and immune therapy have opened up plethora of options to address unmet clinical needs with greater role in haemato-oncology field with promising clinical success in combating graft vs. host disease in transplant patients as well as in preventing infections in them. However, achieving the optimal numbers and the desired phenotypes of cells by conventional expansion culture methods have been major constraints. The increasing use of cell therapy towards myriads of clinical conditions has led to production processes in accordance with Good Manufacturing Practice (GMP) for Advanced Therapeutic Medicinal Product (ATMP). In cellular therapy, safety remains of paramount importance and refers to consistency, quality and potency, not only at the batch release level but also during the process development which should be adapted to closed systems that are easy to use. Implementing dynamic controls during the manufacturing of clinical-grade cells for therapy is essential to ensure microbiological safety and to avoid potential adverse effects linked to genomic instability driving transformation and senescence or decrease of cell functions (immunoregulation, differentiation potential). To meet this growing need of producing required cells of choice in bulk from bone marrow, peripheral blood or other tissues consistently in quality and numbers in a controlled, reproducible, robust, and efficient dynamic environment, tools and technologies have evolved offering in the form of GMP certified closed system at affordable cost to advance cell therapy into practice for the unmet clinical needs. We are at an interesting crossroad to push the limits making it accessible for the best outcomes for patients in precision medicine by validating tools and technologies as well as performing robustly designed clinical trials as way forward.
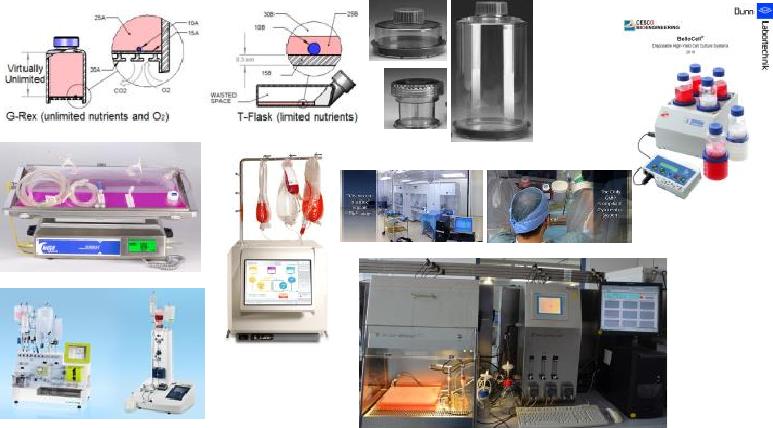
An Overview of Manufacturing possibilities for ATMP Cell Therapy
Guanggang Qu
Shandong Binzhou Animal Science and Veterinary Medicine Academy, China
Title: Efficient expression of porcine circovirus type 2 virus-like particles in Escherichia coli
Time : 14:30-14:55

Biography:
Guanggang Qu is interested in Preventive Veterinary Medicine, Veterinary Biotechnology and Biological Products. He is particularly interested in developing innovated vaccines by E. coli expression system. Currently, he is an Assistant Professor at Shandong Binzhou Animal Science & Veterinary Medicine Academy and Vice Director at Shandong Binzhou Research and Development Centre of propolis vaccine for livestock and poultry. He was a Visiting Scientist at Bhabha Atomic Research Centre and at U.S. Department of Agriculture of USA from 2011 to 2013. He completed his Doctoral Degree at Jilin University, China.
Abstract:
Porcine circovirus 2 (PCV2) is considered to be the etiologic agent responsible for porcine circovirus associated disease (PCVAD) that frequently affects growing pigs at 5-18 weeks of age and can lead to significant negative impacts on profitability of pork production. The capsid (Cap) protein of PCV2 is a major candidate antigen for development of recombinant vaccine and has been successfully used as a vaccine to control porcine circovirus associated disease (PCVAD). In our study, full-length ORF2 gene with codon-optimized for E. coli was synthesized and inserted into the PTF9 (+) expression vector to improve expression of recombinant Cap protein (rCap). A large amount of soluble rCap protein was obtained. The recombinant Cap protein expressed by Escherichia coli (E. coli) has the ability to self-assemble into virus-like particles (VLPs) in vitro; it is particularly an opportunity to develop the PCV2 VLPs vaccine in E coli. In this report, a highly soluble Cap-tag protein expressed in E coli was constructed with a p-TF9 expression vector with a fusion tag of TF9. The recombinant Cap was purified using Ni2+ affinity resins and the tag was removed by the TEV protease. Simultaneously, the whole native Cap protein was able to self-assemble into VLPs in vitro when viewed under an electron microscope. The Cap-like particles had a size and shape that resembled the authentic Cap. The result could also be applied in the large-scale production of VLPs of PCV2 and could be used as a diagnostic antigen or a potential VLP vaccine against PCV2 infection in pigs. We have, for the first time, utilized the Fh8 fusion motif to successfully express the complete Cap protein of PCV2 in E coli. After the cleavage of the fusion motif, the nCap protein self-assembled into VLPs, which can be used as a potential vaccine to protect pigs from PCV2-infection.
Joseph L Mathew
Postgraduate Institute of Medical Education and Research, India
Title: Comparison of susceptibility to measles in preterm infants versus term infants
Time : 14:55-15:20

Biography:
Statement of the Problem: In India and many other developing countries, a single dose of measles vaccine is administered to infants at 9 months of age. This is based on the assumption that maternal transplacentally transferred antibodies will protect infants until that age. However, our previous data showed that most infants lose maternal anti-measles antibodies before 6 months of age, making them susceptible to measles before vaccination at 9 months. This prospective study was designed to compare susceptibility in pre-term vs. term infants, at different time points.
Methodology: Following Institutional Ethics Committee approval and a formal informed consent process, venous blood was drawn from a cohort of 45 consecutive term infants and 45 consecutive pre-term infants (both groups delivered by the vaginal route); at birth, 3 months, 6 months and 9 months (prior to measles vaccination). Serum was separated and anti-measles IgG antibody levels were measured by quantitative ELISA kits (with sensitivity and specificity >95%). Susceptibility to measles was defined as antibody titre <200 mIU/ml. The mean antibody levels were compared between the two groups at the four time points.
Findings: The mean gestation of term babies was 38.5±1.2 weeks; and pre-term babies 34.7±2.8 weeks. The respective mean birth weights were 2655±215 g and 1985±175 g. Reliable maternal vaccination record was available in only 7 of the 90 mothers. Mean antimeasles IgG antibody (±SD) in terms babies was 3165±533 IU/ml at birth, 1074±272 IU/ml at 3 months, 314±153 IU/ml at 6 months and 68±21 IU/ml at 9 months. The corresponding levels in pre-term babies were 2875±612 IU/ml, 948±377 IU/ml, 265±98 IU/ml and 72±33 IU/ml at 9 months (p>0.05 for all inter-group comparisons). The proportion of susceptible term infants at birth, 3 months, 6 months and 9 months was 0%, 16%, 67% and 96%. The corresponding proportions in the pre-term infants were 0%, 29%, 82% and 100% (p>0.05 for all intergroup comparisons).
Conclusion & Significance: Majority of infants are susceptible to measles before 9 months of age suggesting need to anticipate measles vaccination, but there was no statistically significant difference between the proportion of susceptible term and pre-term infants, at any of the four time points. A larger study is required to confirm these findings and compare sero-protection if vaccination is anticipated to be administered between 6 and 9 months.
Abstract:
Joseph L Mathew works at the Advanced Pediatrics Centre, Postgraduate Institute of Medical Education and Research, Chandigarh, India. He has contributed extensively to evidence-based policy-making for several vaccines in the Indian context, especially Hepatitis B, Hib, IPV, MMR, PCV, Influenza, Varicella, acellular pertussis, HPV, Rotavirus, and typhoid conjugate vaccines. He is one of the first to identify the rapid waning of maternal measles antibodies in infancy, creating a pool of susceptible infants/children. He has nearly 200 peer-reviewed publications to his credit and delivered numerous presentations related to vaccinology in national and international meetings.
Aamir Shaikh
Assansa, India
Title: Vaccines for key unmet Medical (Infectious Disease) needs in the developing world: Learning and reflections from the India Polio eradication success story
Time : 15:20-15:45

Biography:
Aamir Shaikh is a “Pharmaceutical Physician” who began his health care industry career in 1998 as a Medical Advisor, with Pfizer India. His early experience with vaccines was related to educational and promotional endeavors with a Hepatitis B vaccine in India. Over the next decade, he successfully delivered in positions of increasing responsibility and leadership, culminating in his role as Director, Medical Affairs & Research for Pfizer India. In 2007, he moved on from Pfizer to start his own small Health Care Consultancy, Assansa. Throughout his health care career, and now through Assansa, his key endeavor is “Helping Health Care Professionals Grow.” He believes this can be achieved through an identified personal philosophy and mindset - one which embraces knowledge, science, business, and ethics.
Abstract:
Over the 20th century, vaccination has been the most effective medical intervention to reduce morbidity and mortality caused by infectious diseases. It is estimated that vaccines save around 2–3 million lives per year worldwide. However, even today, we do not have vaccines to prevent many noteworthy infectious diseases, and access issues preclude available vaccines from being utilized to their fullest potential. Key unmet medical (infectious disease) needs, more so for the developing world, include HIV, malaria, TB, dengue, and others. The Association of Southeast Asian Nations (ASEAN) is responding to these unmet needs through the efforts of the ASEAN-Network for Drugs, Diagnostics, Vaccines, and Traditional Medicines Innovation (ASEAN-NDI). This is important considering that infectious tropical diseases remain prevalent, emerging, and reemerging in the region. India has invested significant health resources towards its ambitious Polio Eradication Program, and is now enjoying the fruits of success. Effectively using the Oral Polio Vaccine (OPV), the key pillars of the Polio Eradication strategy, have visibly demonstrated effective results, and have provided us with useful learning. Moreover, the innovative creation and use of Social Mobilization Network (SMNet) has also provided us with important conceptual and practical lessons for health communication, social mobilization, and for partnerships in global health. Over 2017, and beyond, India will have to grapple with significant challenges - these will need to be addressed with a sense of vigilance and perseverance. There is reason to hope that the challenges of infectious diseases in the 21st century will be adequately addressed by the research, development, access and use of vaccines. Beyond the science, success will largely depend on the “human connect”, and will be facilitated through shared vision, common purpose, effective partnerships, and sound leadership.
Liliya Pekova
University hospital (Stara Zagora), Bulgaria
Title: Pneumococcal meningoencefalitis in 50-day-old baby ended with lethal outcome: A case report
Time : 15:45-16:10

Biography:
Liliya Pekova is a Head of Clinic of Infectious Diseases at University Hospital in Stara Zagora, Bulgaria. She is a Chief of Department of Infectious Diseases, Medical Faculty at Trakia University. Her scientific interests include “Viral hepatitis, neuro-infections and tick-borne diseases”. The main topic of her researches is Acute Hepatitis C. She has two specialties: Infectious diseases and Epidemiology. She is interested in area of Vaccines.
Abstract:
Streptococcus pneumoniae (S. pneumoniae) is a usual agent of pneumonia, sinusitis and medial otitis. In children under two-year-old, it appears as a second bacterial agent which is responsible for neuro infections. We presented a case of S. pneumoniae meningoencephalitis as a primary localization in a 50-day-old breast-fed child. The illness began with high temperature and mild catarrh. Three days later convulsions without meningeal signs were demonstrated. Diagnosis was based on cerebral fluid changes and microbiological verification. In spite of triple antibacterial starting suitable to microbial sensitivity, neuro infection completed with lethal outcome. Laboratorial data–low levels of natrium, severe acidosis in conjunction with high level of protein, cells and low glucosis were considered as prognostic signs for unfavorable outcome. The baby was not vaccinated with synflorix because his age was small. Possibilities to use vaccine against S. pneumoniae earlier than usual were discussed.
Marine Meunier
French Agency for Food, Environmental and Occupational Health & Safety, France
Title: Promising results in the assessment of new vaccine candidates against campylobacter in poultry
Time : 16:10-16:35

Biography:
Marine Meunier completed her Master’s in Immunology and Vaccinology and 7-month internship at GSK Vaccine, and PhD at French Agency for Food, Environmental and Occupational Health & Safety (ANSES) in the Laboratory of Ploufragan. The thesis project in which she is involved is a part of European CAMPYBRO project about control of Campylobacter infection in broiler flocks through two-steps strategy: Nutrition and vaccination. She developed the tools to assess the vaccine potential of these antigens (development of an avian vaccine protocol against Campylobacter and ELISA tests). She used molecular biology tools and FLPC for the production and purification of vaccine antigens.
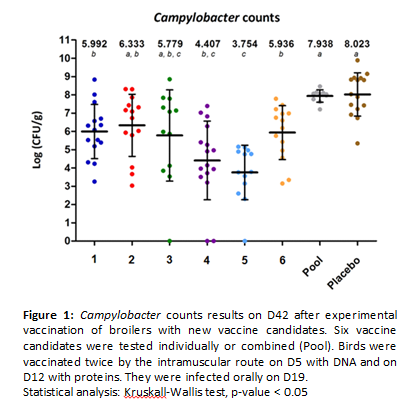
Abstract:
Campylobacteriosis is the most prevalent human bacterial gastrointestinal disease in Europe. Birds are the main reservoir of Campylobacter and human contaminations principally occur by consumption and handling of poultry meat. It was estimated that a Campylobacter reduction from 2 to 3 log10 CFU/g of the intestinal contents in live broilers could be responsible for a decrease of 76% to 100% of the infection in humans. Vaccination of poultry could be a potential way in this goal but despite many studies, no efficient vaccine is available yet and research of more powerful vaccine antigens against Campylobacter is needed. The recent in silico analysis of Campylobacter genome using the reverse volcanology strategy allowed the identification of 14 potential new vaccine candidates. After developing an avian vaccine protocol consisting in two immunizations by the intramuscular route on day five with DNA and day 12 with proteins followed by an oral challenge on day 19, we assessed in vivo immune and protective powers of six new vaccine candidates, individually or in combination. Among the six antigens, four had a significant effect on both IgY production in serum and reduction of Campylobacter caecal counts. The mean reduction of caecal counts with those four proteins varied from 2 to 4.2 log10 CFU/g of caecal content. The pooled proteins had no protective effect. A new in vivo experiment is actually on going in order to confirm these results. The first results are promising since they reach reductions better than those estimated to impact human diseases incidence. Also these results proved the interest of the reverse vaccinology approach to screen new candidate proteins that may become efficient vaccines.